![]()
This post is the third in a series on the origin and history of HeLa S3 cells. The first post details how I came about to ask this question when launching my independent research laboratory. The second post details the life and careers of the legendary physician-scientist pioneer, Dr. Florence Rena Sabin.
Today, we take up a discussion where we will finally learn the origin of HeLa S3 cells, complete with original literature citations.
A recap
We left our previous discussion with the final and still-productive years of Dr. Florence Rena Sabin. After graduating from Johns Hopkins Medical School in 1900, Dr. Sabin embarked on a nearly 40-year career at Hopkins and now-Rockefeller University, elucidating the developmental origin of the lymphatics and antibody responses to tuberculosis and training a generation of physician-scientists. She was truly a pioneer, becoming the first woman to be appointed to faculty at Johns Hopkins, their first female full professor, the first female full member (full prof-equivalent) at Rockefeller, and the first woman invited to join the National Academy of Sciences.
Upon her retirement in 1938, she returned to her native Colorado to join her sister, Mary. Near the end of World War II, she was tapped by the Colorado governor to lead a committee that would address existing public health issues in the state that would have to be solved while absorbing a large number of men and women returning from the war to civilian life. Although in her 70s, Sabin was highly effective and shared the 1950 Lasker Award for Public Service. In 1951, the University of Colorado School of Medicine honored her with the dedication of the Florence R. Sabin Research Building for Cellular Biology.
That same fall was a sharp contrast for the Virginia-born tobacco farmer, Henrietta Lacks. She, too, was spending time at Johns Hopkins. But in this case, it was because her life was being cut short at age 31 from an aggressive case of cervical cancer.
In the months before her death on October 4, 1951, cells derived from her tumor had been successfully cultured in the Hopkins laboratory of Dr. George Gey. These cells were called HeLa, so named for the first two letters of Henrietta Lacks's names, and took a place in history as the first human cell line to be continually propagated in culture. They would live on in laboratories not only at Hopkins but around the world, including that of Colorado geneticist, Dr. Theodore "Ted" Puck.
From bacteria and bacteriophage to human genetics
 Ted Puck came to Colorado a few years earlier. His 1994 autobiography in the American Journal of Medical Genetics tells you far more than I can here. Here he shares his situation before the Sabin Building became available:
Ted Puck came to Colorado a few years earlier. His 1994 autobiography in the American Journal of Medical Genetics tells you far more than I can here. Here he shares his situation before the Sabin Building became available:
On my arrival in Denver in 1948, I first became aware of the magnitude of the responsibility which I had undertaken. The total contribution of the medical school to the budget of the Department of Biophysics was $5,000 per year. All the rest was to come from grant funds. I was the only faculty member in the newly formed department which was required to present a major course to the medical students, to institute graduate training leading to the Ph.D. degree in biophysics, to conduct a post-doctoral training program for
M.D.'s and Ph.D's, to conduct a significant research program in biophysical science, and to raise the necessary grant funds for those activities. An old unused lumber room in the basement of the medical school was cleaned out, painted, and transformed into offices and a laboratory for the department.
A native of Chicago, Dr. Puck did both his undergraduate (1937) and graduate training (1940) at the University of Chicago. His doctoral mentor in Physical Chemistry was Dr. James Franck, a physicist who had shared the 1925 Nobel Prize in Physics. Puck's PhD work focused on the atomic transitions in chlorophyll necessary for photosynthesis.
At the end of his PhD, World War II had begun and Puck remained at Chicago as a research associate to work with Dr. O.H. Robertson on the potential of polyethylene vapor to kill airborne microorganisms for the Commission on Airborne Infections of the Office of the Surgeon General of the Army. The relevance of this project during wartime was exemplified by Puck publishing five papers in Science and six papers in J Exp Med on this work. While on this project, he worked with Phil Marcus, a master's student who would later join him in Colorado.
After the war, Puck did formal postdoctoral work on the genetics and kinetics of bacteriophage interactions with bacteria at the California Institute of Technology with Max Delbrück, who later shared the 1969 Nobel Prize in Physiology or Medicine with Alfred Hershey and Salvador Luria (yes, Luria broth). Delbrück was originally a physicist and obviously contributed to Puck's early enthusiasm for "applying physicochemical approaches to problems of biology."
Clonal populations of mammalian cells
Puck's work with phage impressed upon him that studying human genetics would require that similar techniques be developed to manipulate human cells. Because HeLa cells were taken from the tumor of Henrietta Lacks, they contained several populations of cancer cells. Puck and his then-graduate student Phil Marcus and Steven Cieciura were trying to figure out how to grow clonal populations from single cells.
Establishing "clones," or colonies that grew from a single cell, was first accomplished with mouse L929 cells in 1948 by Katherine Sanford and Gwendolyn Likely in the laboratory of Wilton Earle at the National Cancer Institute (JNCI 1948; 9:229). Earle is credited with the isolation and propagation of the first continuously cultured cell line, mouse L cells. This heterogeneous population was originally isolated in October 1940 as an outgrowth of subcutaneous connective tissue from mammary fat pads excised from a 100-day-old C3H/An mouse (JNCI 1943: 4:165). The cells acquired a malignant phenotype in culture and could produce sarcomas when injected back into mice.
Just as an aside, Earle's group acknowledged the cell culture work of Lewis (Science 1935; 81:545) and Fischer and Davidsohn (Nature 1939; 143:436). The 1935 paper from Warren H. Lewis was his presidential address to the American Association of Anatomists where he reported the continuous culture of rat sarcoma cells for 2-4 years, although no photographs or formal data were shown. Albert Fischer and Fanja Davidsohn reported carrying mouse adenocarcinoma cells for up to 12 years. But while both Lewis and Fischer had lengthy careers, I'm unable to find any follow-up literature on these respective cell lines. Hence, the most continually documented work of Earle's group is most deserving of the "first" designation.
Recognizing that L cells came from a heterogeneous cell population, the Earle group worked on isolating single cells and propagating clonal cultures from those cells. At the time, the composition of cell culture medium was not fully refined and attempts at growing out cells from small populations were unsuccessful. Earle's group recognized that using medium "conditioned" by growing cells produced diffusible factors that encouraged growth of small cell populations. To produce single clones, Earle's group painstakingly transferred single L cells to small capillary tubes together with a small amount of conditioned medium, producing the first clonal cultures of immortalized mammalian cells. The most widely studied of these is clone 929, known more properly as NCTC L-929 cells, the first immortal mammalian cell line offered by ATCC, appropriately as CCL-1.
The capillary approach to cloning seemed too cumbersome to Puck and he wanted to develop a method that was more analogous to isolating clonal bacteria cultures from distinct colonies that had grown from individual cells. Moreover, quantitative assessment of cellular sensitivity to drugs or radiation would not be possible using the capillary method. Because he was interested in human genetics, he decided to work with HeLa cells. As cited below in references 1 and 2, he obtained his original HeLa culture from the George Washington Carver Foundation of historically black institution, Tuskegee University in Alabama, rather than from George Gey at Hopkins who had originally isolated the cells.
From Hopkins to Minnesota to Alabama to Colorado
The role of Tuskegee in the polio treatment and vaccination effort is an engaging story in its own right that is told by Rebecca Skloot in her book and worthy of expanding into its own separate post. The National Foundation for Infantile Paralysis (NFIP), known today as the March of Dimes, had funded a HeLa mass production laboratory at Tuskegee to provide a uniform source of cells in tests of the Salk polio vaccine after having had established a hospital there in the late 1930s to care for black polio victims.
William Scherer and Jerome Syverton at the University of Minnesota (together with Gey) had shown that HeLa cells could be infected with all three strains of the poliomyelitis virus circulating at the time which could then be neutralized by incubation with antibodies to the virus (J Exp Med 1953; 97:695). To test the efficacy of the vaccine, 23 sites around the country were charged with taking post-vaccine blood samples from over 2 million children to test the ability of their serum to neutralize the virus in culture as an indication of antibody titer. The Carver Foundation at Tuskegee supplied these cells, but also provided samples to researchers such as Puck. When starting the production facility, Tuskegee scientists Russell W. Brown and James H.M. Henderson traveled to the laboratory Scherer and Syverton at Minnesota to learn HeLa culturing techniques. Scherer was one of the first recipients of HeLa cells from George Gey and in fact had maintained an original culture, something Gey himself had not. So, when ATCC later asked Gey to submit a culture for the repository, Gey had to solicit Scherer. When you look up the original HeLa culture (CCL-2) at ATCC, you will see that Scherer, not Gey, was the submitter.
Clonal isolation of HeLa S3
Puck hypothesized that a highly conditioned medium could be produced by a monolayer of cells that had been rendered incapable to dividing by irradiation. By providing conditioned medium in a large vessel capable of supporting colony growth, it would be easier to quantify the efficiency with which clones were produced while optimizing media composition. He defined such replication-incompetent cells as "feeder layers." In initial experiments with postdoctoral fellow Dr. Roshan Christensen, they attempted UV radiation but it was insufficient to damage the cellular DNA. They were about to move on to X-rays when Christensen became pregnant. She and Puck made the decision that use of X-rays was not in the best interest of her baby and she moved on to other projects until the next person joined the lab.
That next person was Phil Marcus, the MS research associate Puck had known from the University of Chicago. Marcus was recruited by Puck with the intention of having him join the new PhD program at Colorado once he met the course requirements. Around the same time, famed atomic physicist Leó Szilárd was visiting Puck's lab to learn about molecular biology. This is the same Szilárd whose work with Enrico Fermi on the Manhattan Project led to the atomic bomb. As Puck noted in his autobiography, Szilárd began involving himself in the project without advance discussion, instead talking privately with Marcus.
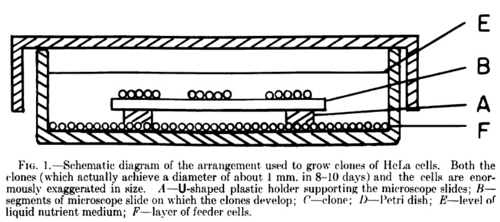
Initially, Puck had wanted the feeder layer to be irradiated and new HeLa cells plated on top of them to grow into colonies in empty spaces on the Petri dish. Szilárd appears to have understood that the feeder layer was necessary but had not known of the idea to irradiate the cells. Therefore, he suggested privately to Marcus that he construct a glass slide platform above a freely growing feeder layer on which single HeLa cells would be plated. Over the course of time, hard feelings developed between the young professor and the senior scientist. The ultimate outcome, however, was the combination of the two ideas: Szilárd's platform above Puck's irradiated HeLa feeder layer.
The drawing above comes from a 1955 PNAS paper by Puck and Marcus (1) where Szilárd's contribution is noted in the acknowledgments. The irradiated feeder layer of cells is at the bottom. The microscope slide showing the colonies generated was originally prepared by plating a dilute suspension of HeLa cells containing only 5 to 100. The cells were allowed to attach to the slide for 5 hours, then placed in the media with the irradiated feeder layer. After 8-10 days of incubation, colonies of 800-2,000 cells grew out from the original plated cells.
But how to harvest the clones? In a subsequent 1956 paper in the Journal of Experimental Medicine (2) Puck, Marcus, and Steven Cieciura describe the use of trypsin to harvest the colonies using what we today call cloning rings. Here is the original text:
This single passage is notable in that it describes not only clonal isolation and propagation of cells but establishes the basis for clonogenic assays of cancer cells (a measure of a given cell's ability to replicate into colonies) and also the more stringent soft-agar assay which measures a cell's ability to form three-dimensional colonies.
And finally after three blogposts, we learn what HeLa S3 cells are: they were the third clone isolated and propagated from the heterogeneous HeLa culture with the letter S in honor of Dr. Florence Rena Sabin, for whom the building housing Puck's new laboratory was named in December, 1951.
Why S3 and not S1 or S238?
But I couldn't stop there. I wanted to know why Puck continued working with the S3 clone and why that is the one deposited with ATCC and sold as CCL-2.2.
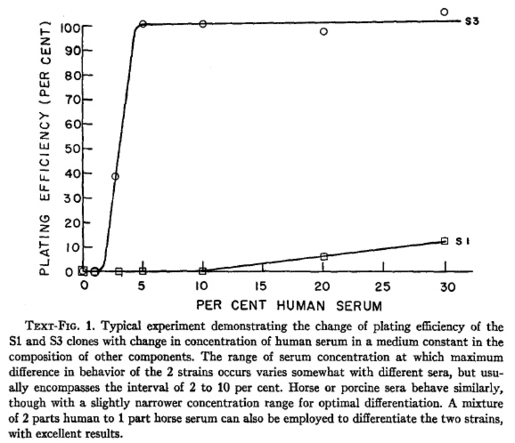
Puck's laboratory continued this work in an attempt to better define media components necessary for growing these cells at high clonogenic or "plating" efficiency. First Puck and Harold Fisher followed the observation that HeLa S1 cells formed smaller colonies that HeLa S3 cells grown for the same number of days. In their 1956 J Exp Med paper (3), they demonstrated below that in the absence of a feeder layer, the S3 clone could still form colonies at very low concentrations of human serum used in the growth medium. (By the way, the human serum was also provided by the Carver Foundation at Tuskegee from who paid local donors with funds from the NFIP contract - we know today that HeLa cells can be grown with fetal calf serum instead).
We know today that the feeder layer "conditioned" the media with peptide growth factors but there were also some important biochemicals they provided that were missing from the media used at the time. In their 1957 Science paper (4), Gordon Sato, Fisher, and Puck showed that HeLa S3 cells required inositol to form colonies in the absence of a feeder layer but were inositol-independent when a feeder layer was present. In other experiments, they showed that the HeLa S3 cells also required cholesterol.
But when they analyzed the series of S clones for their requirements across the board, the S3 clone was the most hardy and grew with the highest plating efficiency under optimized condition. The authors also noted that the S3 phenotype seemed to be the most predominant in the original heterogeneous HeLa cell population.
Today, as we have gone away from the use of high concentrations of human serum toward low(er) concentrations of fetal calf serum, I wonder if mixed HeLa cultures still contain the less robust S1 cells. As I said in an earlier post, the HeLa S3 cells look more compact and uniform than most HeLa cultures I have worked with. But these have been carried separately under varied conditions over the course of decades. So, your choice of HeLa or HeLa S3 cells for your work may be one more of semantics than practicality.
I welcome comments from others who may have personal lab experience with either cell population.
What was so special about HeLa cells?
I know that Rebecca Skloot has gotten this question ad nauseum while on her book tour. It is the obvious question: what about HeLa cells made them the first continuously culturable human cell line out of the hundreds of tumor specimens that came from Johns Hopkins surgical wards and into the laboratory of George Gey?
I thought more about this while reading this old papers. We know today that HeLa cell DNA contains portions of a human papillomavirus genome and it overexpresses telomerase, both mechanisms by which normal cells can be transformed. But many cells are virally-transformed and many more overexpress telomerase. And even though HeLa cells are billed as growing rapidly, their 24 hr doubling time is shared by a great many tumor cell lines.
Instead, I think that the tumor cells from Ms. Henrietta Lacks were somehow capable of tolerating the incomplete nutritional conditions of the cell culture media used in the early 1950s. We know from Puck's work that the cells grow optimally in the presence of inositol and cholesterol but other cell lines have much more stringent requirements for non-essential amino acids, substrates for oxidative phosphorylation, and only tolerate very narrow ranges of pH. I've not conducted any formal comparative investigations but I think that Henrietta Lacks's tumor cells were so aberrant that they had minimal nutritional requirements relative to cells from other tumors that made it to the Gey laboratory.
This, I suspect, is also why Ms. Lacks's cancer was so unique and voracious as described by the pathologist at that time. It grew at metastatic sites that may have been suboptimal in terms of availability of biochemicals and maybe even tolerated hypoxia and/or acidic pH better than other cervical cancers. I know that many labs have new students first try their hand at culturing HeLa cells because they are hardy and forgiving of bad technique.
Perhaps what makes HeLa cells so easy to culture are the same reasons that made them so deadly to Ms. Lacks.
I have more to say about Dr. Ted Puck because he was truly a remarkable scientist and gentleman. He made many other contributions in his 50+ year career and I'd like to share that with you in another post.
But now you know the difference between HeLa and HeLa S3.
References:
(1) Puck TT and Marcus PI (1955). A rapid method for viable cell titration and clone production with HeLa cells in tissue culture: the use of X-irradiated cells to supply conditioning factors Proceedings of the National Academy of Sciences, 41 (7), 432-437 DOI: 10.1073/pnas.41.7.432
(2) Puck TT, Marcus PI, and Cieciura SJ (1956). Clonal growth of mammalian cells in vitro: growth characteristics of colonies from single HeLa cells with and without a "feeder" layer Journal of Experimental Medicine, 103 (2), 273-284 DOI: 10.1084/jem.103.2.273
(3) Puck TT and Fisher HW (1956). Genetics of somatic mammalian cells: I. Demonstration of the existence of mutants with different growth requirements in a human cancer cell strain HeLa Journal of Experimental Medicine, 104 (3), 427-434 DOI: 10.1084/jem.104.3.427
(4) Sato G, Fisher HW, and Puck TT (1957). Molecular growth requirements of single mammalian cells Science, 126 (3280), 961-964 DOI: 10.1126/science.126.3280.961











Comments
please consider submitting your post(s) about Dr Sabin to the Diversity in Science carnival for March, celebrating women's history month.
Posted by: DNLee | March 16, 2010 11:24 PM
Hela cells were not the first to be immortalized in culture. Read hayflicks letter to science for details(latest issue).
Posted by: Canada | March 17, 2010 12:38 PM
Should the title say Ted Puck, PhD?
Posted by: MitoScientist | March 17, 2010 4:06 PM
@DNLee - Hello and congratulations to the newly-minted Dr. Lee!!! Yes, indeed, I'll submit the previous post to the Diversity in Science Carnival for Women's History Month.
@Canada - I believe that you are referring to a letter from Dr. Hayflick to Nature about Steve Silberman's review of The Immortal Life of Henrietta Lacks. Mr. Silberman simply left out the word "human" from his description of HeLa as the first immortal line.
As Dr. Hayflick pointed out, and as I wrote about extensively above, the first immortal cell line was the mouse L cell line isolated by Wilton Earle in late 1940, published in 1943.
@MitoScientist - Yes, you are correct. I will make the change to PhD in the title. Thanks for catching that.
Posted by: Abel Pharmboy | March 17, 2010 5:50 PM
Many, many thanks for reminding me of the origin of the cells which I have worked with daily for over 30 years. Very well done.
Posted by: Vincent Racaniello | March 18, 2010 9:25 PM
For the record, I didn't leave anything out of my Nature review of Skloot's wonderful book. The Nature editors left the word "human" out of the subhead, that's all.
Posted by: Steve Silberman | March 20, 2010 6:38 PM
Thank you Professor Racaniello for your kind words. Your work on books, podcasts, and other outreach is an inspiration to me - I've learned a great deal of virology from you and use some of your materials in my classes. It was a delight to go back and read some of these old papers and think what it was like to do this work at the time.
Steve, that's a very good point, one that all readers should note. While I have the luxury of titling my posts whatever I want, print writers are generally at the mercy of editors when titles are selected for their articles.
So, I stand corrected on my comment above - Mr. Silberman did not leave out "human." The editor did. I hope that information makes its way back to Dr. Hayflick.
Posted by: Abel Pharmboy | March 21, 2010 11:11 AM